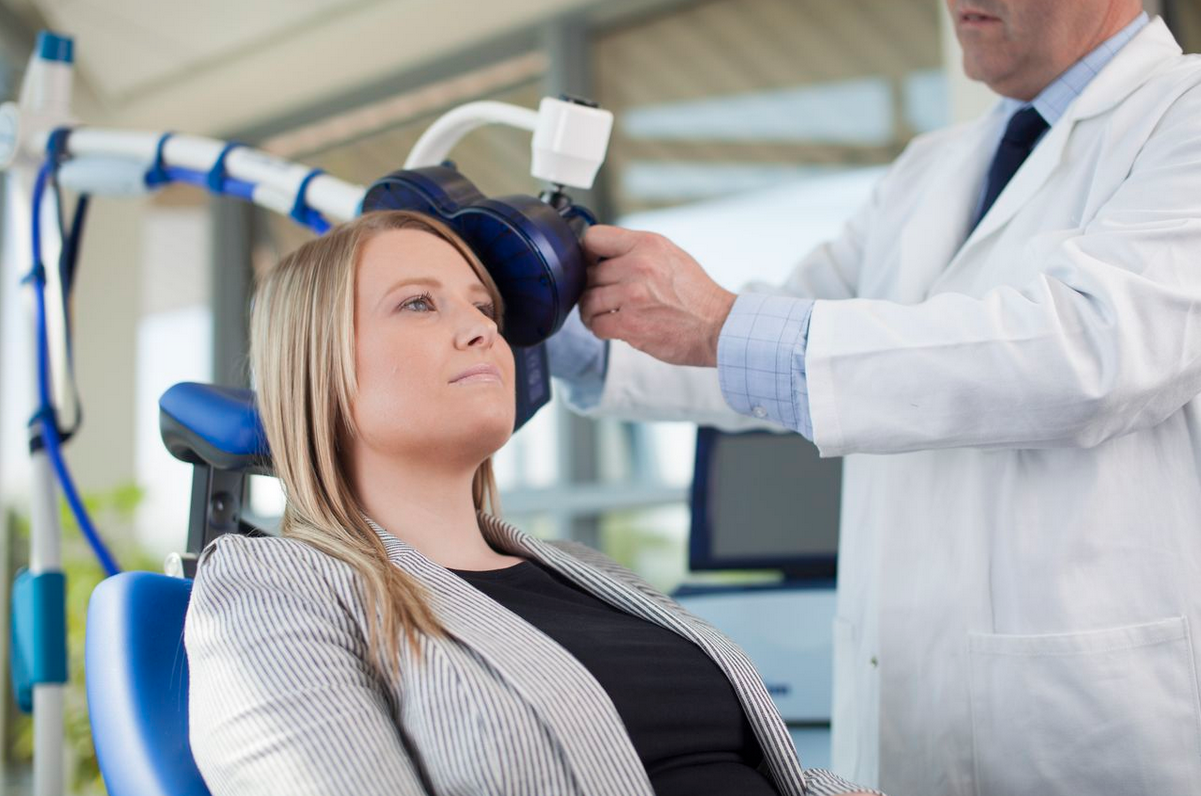
Meet rTMS: Repetitive Transcranial Magnetic Stimulation
One treatment modality gaining favor in the Psychiatric care world is Repetitive Transcranial Magnetic Stimulation ( rTMS ). TMS is part of a group of treatments called Interventional Psychiatry that also include ketamine therapy and ECT. Approved by the FDA in 2008, rTMS is a safe, non-invasive neuromodulation therapy directed at patients who suffer from Major Depressive Disorder (MDD). Much of the current research involved with studying the efficacy of rTMS relates to refining the predictive success of rTMS treatment as it relates to patients’ attributes that may assist in determining which patients will respond to the treatment.
A related but different type of treatment is dTMS, that uses deeper and multi-point stimulation. dTMS has been FDA approved since 2013.
Application and Results
rTMS therapy uses stimuli applied to the prefrontal cortex that induces a magnetic field. In turn, these stimuli have the capacity to modulate circuits in the brain relating to emotion regulation and depressive symptoms. The treatment involves fitting a coil that sits gently on a patient’s scalp to administer the magnetic pulses. The physician will calibrate the appropriate strength of pulses based on each patient’s sensitivity. At present, there are five approved devices used for rTMS under different brand names.
One impediment for the treatment to have wider application is that rTMS requires a significant commitment of time and investment on the part of patients. Treatments are administered daily (or almost daily) for 20-30 sessions, and a course of treatment can cost as much as $12,000. rTMS treatments are not “one size fits all,” and there are a number of variables for a physician to consider, including the type of coil, location of the stimulus, stimulus intensity, and stimulus frequency.
rTMS has been used successfully in instances where a patient has not responded to conventional medical treatments, and the best results have been achieved with patients who have failed only one medical trial versus those who have failed two or more different trials. Indeed, current research indicates that for patients who have tried and failed two courses of antidepressant treatments, the chances of achieving and maintaining remission with rTMS is very low.
Optimizing Outcomes
Better outcomes have also been achieved with patients with lower levels of anxiety (although rTMS can be successful in cases where a patient does have significant comorbid anxiety,) lower levels of psychosis, and a longer duration of their current episode of depression. In instances where a patient exhibits psychotic symptoms or acute suicidal ideation, rTMS should not be used and the patient and psychiatrist will most likely select another treatment.
In order to be considered for rTMS treatment, a comprehensive medical screening is necessary. Patients with certain types of implants, including pacemakers, implantable cardioverter defibrillators (ICDs), vagus nerve stimulators, should not receive rTMS. In clinical trials with over 10,000 applications, there have been comparatively few side effects from rTMS treatment, with minor scalp discomfort reported most commonly.
For patients with depression that has not responded to antidepressant medications, I would be delighted to discuss alternative forms of treatment, including rTMS.
For additional information, please feel free to email our office at Amanda.Itzkoff@gmail.com. To schedule an appointment, call our offices at 917-609-4990.