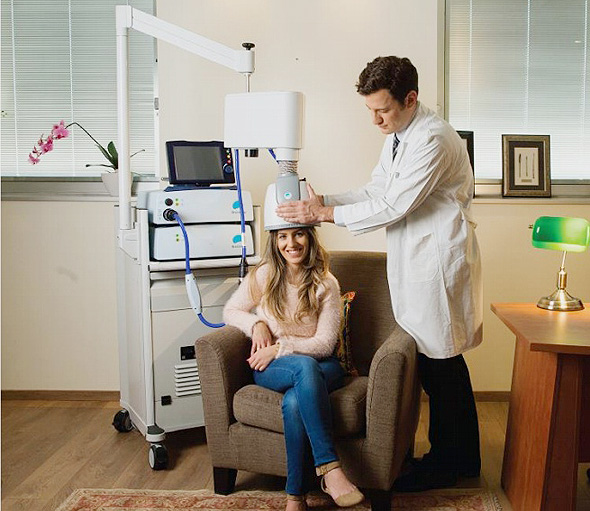
Deep TMS or ‘dTMS’, a form of transcranial magnetic stimulation that addresses deeper lying brain pathways than its sister treatment rTMS, is continuing to impress practitioners and their patients heading into 2020.
BrainsWay dTMS is a non-invasive treatment that safely and effectively targets regions of the brain to combat the effects of major depressive disorder (MDD). The focus for TMS modalities are patients who have not responded to other treatment methods such as antidepressants (SSRIs).
Recent dTMS Research
A recent study involving 228 patients and funded independent of corporate sponsorship indicated significantly lower remission rates of depression for both dTMS and rTMS interventions. dTMS results after a four-week session period noted a reduction of 59.7% versus 42.7 for rTMS.
The depression scale used for the study—the HAM-D17-was lowered by 51% for the dTMS study group and 41% for the rTMS group.
The results were published in the July, 2019 issue of the Journal of Psychiatric Research. Academic observers noted that the results were important due to the fact that his is the first time different TMS technologies have been compared.

Other dTMS Breakthroughs on the Horizon
While MDD has been the focus for dTMS, the treatment holds promise for other disorders such as obsessive-compulsive disorder (OCD), smoking cessation, and posttraumatic stress disorder (PTSD).
BrainsWay is planning a new round of clinical trials for complex addiction and pain-related problems. This emerging group includes MS-related pain, opioid addiction, and rehabilitation for stroke victims.
dTMS is an FDA approved treatment method that provides relief for depression and other symptoms. Clinical research continues to provide highly positive results, and BrainsWay dTMS is pioneering new areas of research.
